by Epica-MKTG | Jun 29, 2024 | News
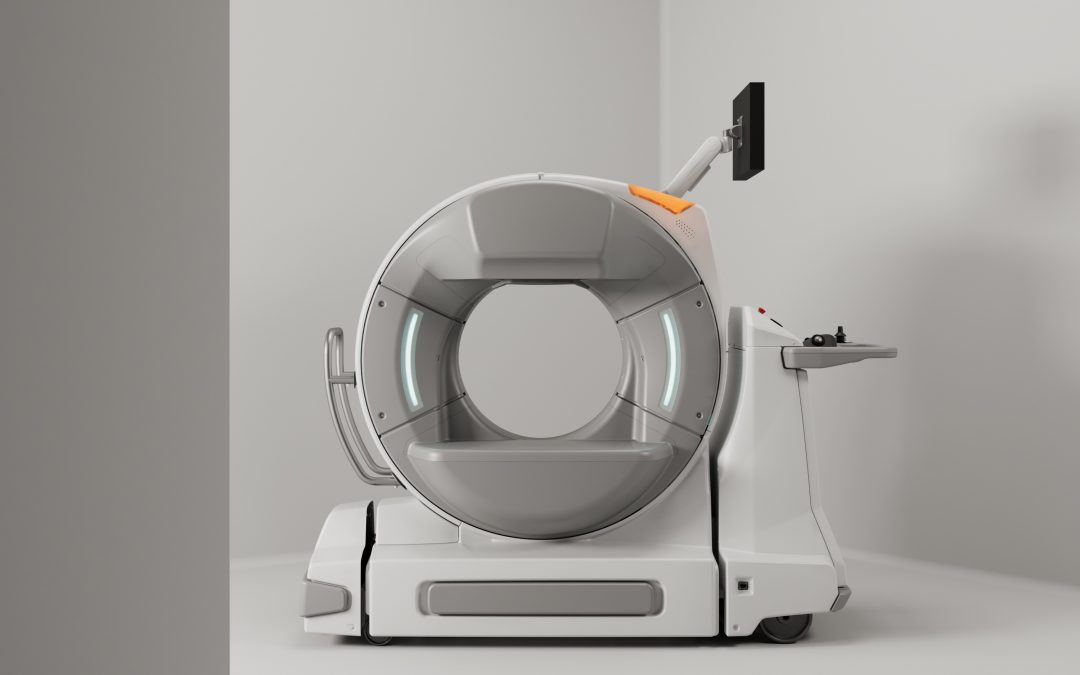
Epica International is thrilled to announce a significant milestone: the FDA has granted 510(k) clearance for expanded use of our SeeFactor CT3™ imaging system. This new clearance enhances the system’s capabilities, making it an even more indispensable tool for...



Recent Comments